Pd-catalyzed access to mono- and di-fluoroallylic amines from primary anilines†
Abstract
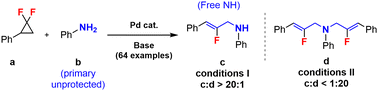
The Pd-catalyzed highly selective synthesis of mono- and di-2-fluoroallylic amines from gem-difluorocyclopropanes and ubiquitous unprotected primary anilines is herein described. Initial kinetic investigations suggest a first order in the gem-difluorocyclopropane substrate, as well as a circa zeroth order in the aniline coupling partner. The newly produced fluoroallylic motifs should find important applications in synthetic as well as medicinal chemistry and stimulate the further development of coupling methods based on strained cyclic building blocks.


 Please wait while we load your content...
Please wait while we load your content...