Ketone-derived 2,3-dihydroquinazolinones in N-heteroarene C–H alkylation via C–C bond scission under oxidative metal catalysis†
Abstract
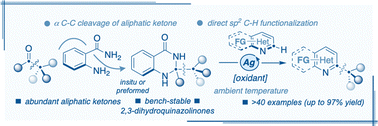
A silver-catalysed oxidative sp2 C–H alkylation of N-heteroarenes with ketone-derived 2,3-dihydroquinazolinones at room temperature is developed. The combination of a metal catalyst and perdisulfate oxidant promotes the rarely explored thermal activation of pre-aromatic 2,3-dihydroquinazolinone to generate an alkyl radical, supported by mechanistic studies. In addition to the broad scope, good functionality tolerance, late stage functionalization of APIs, and synthesis of a novel Papaverine analogue, the utilization of an N-heteroarene C–H bond and ketone as a non-trivial alkyl radical source represents the salient feature of this method.
- This article is part of the themed collection: Chemical Communications HOT Articles 2022


 Please wait while we load your content...
Please wait while we load your content...