A general nickel-catalyzed highly regioselective hydroarylation of unactivated alkenes enabled by the picolinamide auxiliary†
Abstract
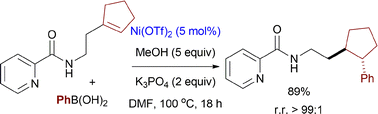
A practical method for regioselective hydroarylation of unactivated γ- or δ-vinyl alkylamines has been reported, enabling facile preparation of highly value-added ε- or ζ-aryl alkylamines. The protocol employs nickel catalysis, shows high functional group tolerance and can be used for modifying bio-related molecules.


 Please wait while we load your content...
Please wait while we load your content...