Palladium-catalyzed oxidative Heck reaction of non-activated alkenes directed by fluorinated alcohol†
Abstract
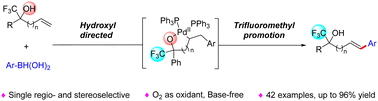
A novel, Pd-catalyzed oxidative Heck reaction of non-activated alkenes synergistically directed by bifunctional groups has been developed firstly by using O2 as a green oxidant, yielding the oxidative Heck products with excellent yields in a regio- and stereoselective manner. This bifunctional synergistic activation mechanism was demonstrated by experimental analysis and detailed computational studies, wherein the hydroxyl group directs the migratory insertion of the alkenes and the trifluoromethyl group facilitates the subsequent β-H elimination and reductive elimination. Moreover, a pesticidal active compound was synthesized using the bifunctional synergistically directed C–H arylation as the key step, which demonstrated its synthetic utility.


 Please wait while we load your content...
Please wait while we load your content...