Homogeneous catalysis of dioxygen reduction by molecular Mn complexes
Abstract
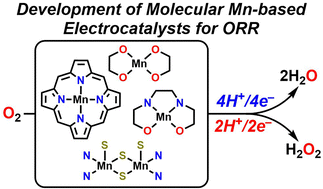
Continually increasing global energy demand perpetuates the need for effective alternative energy sources and ‘green’ industrial processes. The oxygen reduction reaction (ORR) is crucial to the development of hydrogen fuel cells, a key device in the development of alternative energy sources. Further, the ORR to hydrogen peroxide by electrochemical means can provide an environmentally friendly alternative to its industrial production, which is capital and energy intensive. While Pt has traditionally been the best electrocatalyst for the ORR, inspiration from active sites in nature that bind and transport O2 has led to the development of earth-abundant transition metal catalysts. However, despite the prevalence of Mn-based active sites that bind and activate O2 in biological systems, there remains a lack of developed Mn-centered catalysts for ORR in comparison to Fe and Co. Here, we summarize known Mn-based molecular electrocatalysts for the ORR and describe their activity as well as future directions of the field.


 Please wait while we load your content...
Please wait while we load your content...