Photoredox-catalysed chlorination of quinoxalin-2(1H)-ones enabled by using CHCl3 as a chlorine source†
Abstract
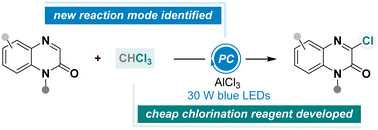
A photoredox-catalysed chlorination of quinoxalin-2(1H)-ones was developed by using CHCl3 as a chlorine source, thus affording various 3-chloroquinoxalin-2(1H)-ones in moderate to high yields. This protocol is characterized by mild reaction conditions, excellent regioselectivity, and readily available chlorination agent. Considering the operational simplicity and low cost of this chlorination approach, this developed method offers an innovative pathway for rapid incorporation of chlorine functionality into heteroarenes, and will inspire broader exploitation of new chlorination strategies.


 Please wait while we load your content...
Please wait while we load your content...