Organocatalytic multicomponent coupling to access a highly functionalised tetracyclic furoindoline: interrupted Passerini/Joullié–Ugi cascade reaction†
Abstract
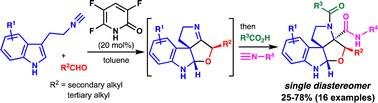
The interrupted Passerini reaction of 3-(2-isocyanoethyl)-indole catalysed by 3,5,6-trifluoro-2-pyridone is described. The reaction diastereoselectively provided a tetracyclic furolindoline, which proved to be a good substrate for the Joullié–Ugi reaction; therefore, the sequential Passerini/Joullié–Ugi reactions were performed in one-pot to rapidly provide versatile and highly functionalised furoindolines from 3-(2-isocyanoethyl)-indole.


 Please wait while we load your content...
Please wait while we load your content...