Diastereoselective access to substituted oxetanes via hydrosilylation–iodocyclisation of homopropargylic alcohols†
Abstract
The regio and stereoselective hydrosilylation of a variety of homopropargylic alcohols and their derivatives is described. The reaction is tolerant to a variety of sterically and electronically varied substrates, affording only the E-vinyl silane as a sole regioisomer. The application of the resultant vinyl silanes towards the diastereoselective synthesis of tetrasubstituted oxetanes is demonstrated.
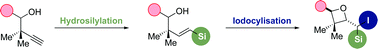


 Please wait while we load your content...
Please wait while we load your content...