Copper metal electrode reversibly hosts fluoride in a 16 m KF aqueous electrolyte†
Abstract
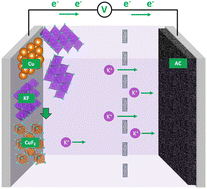
Fluoride is a promising charge carrier for batteries due to its high charge/mass ratio and small radius. Here, we report commercial copper powder exhibits a reversible capacity of up to 222 mA h g−1 in a saturated electrolyte of 16 m KF. This electrolyte suppresses dissolution of CuF2, the charged product. Furthermore, the KF solid comprised in the Cu electrode facilitates a high initial capacity. Our results showcase the potential of aqueous fluoride batteries using copper as an electrode.


 Please wait while we load your content...
Please wait while we load your content...