Cobalt-catalyzed C(sp2)–H bond imination of phenylalanine derivatives†
Abstract
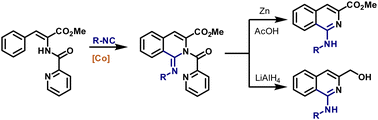
Herein we report the cobalt-catalyzed, picolinamide-directed C–H bond imination protocol of phenylalanine derivatives using isocyanides and a Co(dpm)2 catalyst. A wide range of functional groups were tolerated under the reaction conditions, yielding imines in high yields. The obtained imine products can easily be transformed to 1-aminoisoquinoline derivatives under reductive conditions, providing an attractive alternative to already existing methodologies. The control experiments indicated that C–H activation might occur via an electrophilic pathway.


 Please wait while we load your content...
Please wait while we load your content...