Computational insights into strain-increase allylborations for alkylidenecyclopropanes†
Abstract
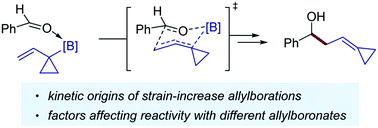
The origins of the reactivity of strain-increase allylborations were computationally investigated. The low reactivity of vinylcyclopropyl boronates is due to weak electronic interactions between benzaldehyde and allylboronates. By increasing the acidity of the boron center, the reactivity is significantly improved because the stronger stabilizing O→B interaction effectively compensates for destabilizing steric effects.


 Please wait while we load your content...
Please wait while we load your content...