Ru(II)-catalyzed P(III)-assisted C8-alkylation of naphthphosphines†
Abstract
We report a phosphine-directed ruthenium-catalyzed C8-selective alkylation of naphthalenes with alkenes. This protocol provides straightforward access to a large library of electron-rich C8-alkyl substituent 1-naphthphosphines, which outperformed common commercial phosphines and their precursors in the Pd-catalyzed Suzuki–Miyaura coupling of aryl bromides with alkylboronic acid.
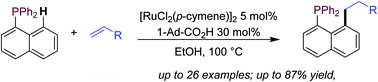


 Please wait while we load your content...
Please wait while we load your content...