Isothiourea-catalyzed formal enantioselective conjugate addition of benzophenone imines to β-fluorinated α,β-unsaturated esters†
Abstract
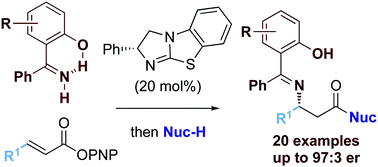
The isothiourea-catalyzed formal enantioselective conjugate addition of 2-hydroxybenzophenone imine derivatives to α,β-unsaturated para-nitrophenyl esters has been developed. Investigations of the scope and limitations of this procedure showed that β-electron withdrawing substituents within the α,β-unsaturated ester component are required for good product yield, giving rise to a range of β-imino ester and amide derivatives in moderate to good isolated yields with excellent enantioselectivity (20 examples, up to 81% yield and 97 : 3 er).
- This article is part of the themed collection: Celebrating 10 years of ChemComm Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...