Asymmetric cyclopropanation of electron-rich alkenes by the racemic diene rhodium catalyst: the chiral poisoning approach†
Abstract
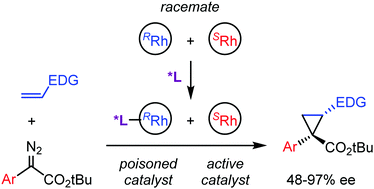
Asymmetric cyclopropanation of alkenes by aryldiazoacetates was achieved using the readily-available racemic (diene)rhodium complex in combination with the chiral oxazoline-phenol ligand, which acts as the chiral poison and selectively inhibits one of the enantiomers of the catalyst. This approach eliminates a common problematic step of the synthesis of chiral catalysts.


 Please wait while we load your content...
Please wait while we load your content...