Enantioselective transannular reactions by palladium-catalysed conjugate addition of aryl boronic acids†
Abstract
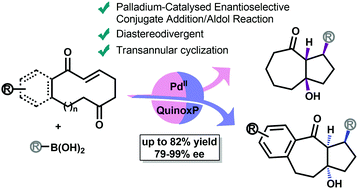
A palladium-catalyzed asymmeric conjugate addition of aryl boronic acids to medium-sized cycloalkenones followed by intramolecular aldol trapping is reported. The use of in situ formed [Pd/(QuinoxP*)] as the catalyst enables the synthesis of arylbicyclic scaffolds in good yields and with excellent stereocontrol (up to 7 : 1 dr, up to 99% ee). The reaction is applicable to a range of medium size ketoenone substrates and funcionalized aryl boronic acids, including heterocyclic compounds.


 Please wait while we load your content...
Please wait while we load your content...