Unearthing the unique stability of thiophosphonium-C-terminal cysteine adducts on peptides and proteins†
Abstract
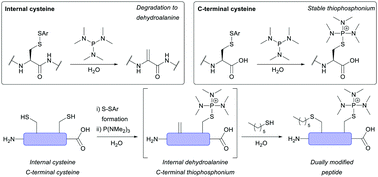
Herein we report a fundamental discovery on the use of tris(dialkylamino)phosphine reagents for peptide and protein modification. We discovered that C-terminal thiophosphonium species, which are uniquely stable, could be selectively and rapidly generated from their disulfide counterparts. In sharp and direct contrast, internal thiophosphonium species rapidly degrade to dehydroalanine. We demonstrate this remarkable chemoselectivity on a bis-cysteine model peptide, and the formation of a stable C-terminal-thiophosphonium adduct on an antibody fragment, as well as characterise the species in various small molecule/peptide studies.
- This article is part of the themed collection: Chemical Communications HOT Articles 2022


 Please wait while we load your content...
Please wait while we load your content...