Total synthesis of streptovertidione and bioinspired transformation to streptovertidine A and formicapyridine A†
Abstract
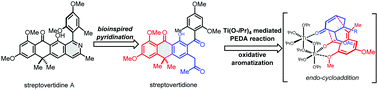
We report herein a concise total synthesis of streptovertidione, and its transformation to streptovertidine A and formicapyridine A through a bioinspired pyridination. This strategy features: (1) a one-pot Ti(O-iPr)4-mediated photoenolization/Diels–Alder (PEDA) reaction/oxidative aromatization sequence for the construction of gem-dimethyl-anthracenone, a naturally occurring antibiotic pharmacophore; (2) a late-stage pyridination based on the biosynthetic hypothesis. This efficient route supports the preparation of other formicapyridines and derivatives.
- This article is part of the themed collection: 2022 Pioneering Investigators


 Please wait while we load your content...
Please wait while we load your content...