A tandem process for the synthesis of β-aminoboronic acids from aziridines with haloamine intermediates†
Abstract
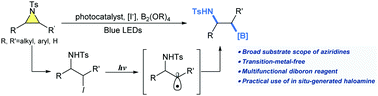
An unprecedented synthetic strategy is devised to generate β-aminoboronic acids from aziridines via a sequential process involving 1,2-iodoamine formation and radical borylation under light irradiation. A variety of aziridines including multiply substituted aziridines have been successfully employed as synthetic precursors, expanding their synthetic utility compared to previous methods. Mechanistic studies suggest that the boron source plays a unique role in the borylation step, and in the formation of haloamine intermediates.
- This article is part of the themed collection: 2022 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...