Phosphine-catalyzed hydroboration of propiolonitriles: access to (E)-1,2-vinylcyanotrifluoroborate derivatives†
Abstract
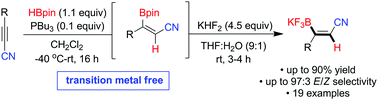
We report an organocatalytic trans hydroboration of 3-substituted-propiolonitriles. In the presence of catalytic amounts of tributylphosphine and pinacolborane, regioselective hydroboration of the internal triple bond proceeded in a stereoselective fashion under mild conditions to afford the corresponding (E)-1,2-vinylcyanoborane derivatives. The mechanism is proposed to occur through a 1,2-phosphine addition instead of a canonical 1,4-conjugate addition pathway.
- This article is part of the themed collection: Celebrating 10 years of ChemComm Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...