Increasing the oxidation power of TCNQ by coordination of B(C6F5)3†
Abstract
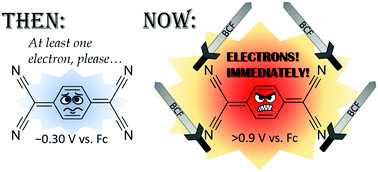
The oxidation power of the cyanocarbon TCNQ (tetracyano-quinodimethane) can be significantly increased to approximately E = +0.9 V vs. Cp2Fe by coordination of up to four equivalents of the strong fluorinated Lewis acid B(C6F5)3, resulting in a highly reactive but easy-to-use oxidation system. Thianthrene and tris(4-bromophenyl)amine were oxidized to the corresponding radical cations. Dianionic [TCNQ·4 B(C6F5)3]2− was formed upon reduction with two equivalents of ferrocene or decamethylcobaltocene. [TCNQ·4 B(C6F5)3]− and [TCNQ·4 B(C6F5)3]2− are rare cases of redox-active weakly-coordinating anions.


 Please wait while we load your content...
Please wait while we load your content...