Tuning the aromatic backbone twist in dipyrrolonaphthyridinediones†
Abstract
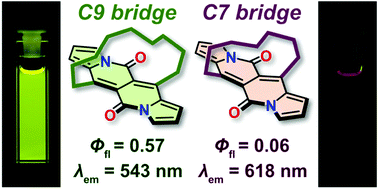
This communication describes the photophysical behavior of three analogs of cyclophane bearing the dipyrrolonaphthyridinedione (DPND) core. In these molecules, intersystem crossing (ISC) can be successfully induced by distinct changes in the deviation from planarity within the DPND core, allowing at the same time the emission maximum to shift from the green to red region of the visible spectrum without any synthetic modifications of the chromophore structure. This finding may build the foundation for a new paradigm for inducing ISC-type transitions within other centrosymmetric and planar cross-conjugated chromophores.


 Please wait while we load your content...
Please wait while we load your content...