Grignard reagent formation via C–F bond activation: a centenary perspective
Abstract
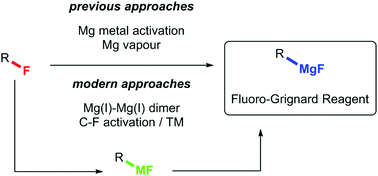
Examples of Grignard reagents obtained by C–F bond activation with magnesium have kept appearing in the literature over the last century. Due to the high bond dissociation energy of the C–F bond, a lot of effort has been invested in the preparation of highly active forms of magnesium for this purpose. Originally, magnesium activation was achieved by the application of additives, notably iodine. Later work focused on the generation of highly active magnesium powder by reduction of magnesium salts with alkali metals (“Rieke magnesium”). Modern approaches to the problem involve the application of Mg(I)–Mg(I) dimers and C–F bond activation performed by a transition metal catalyst followed by transmetallation with a magnesium salt. The purpose of this article is to provide an overview of fluoro-Grignard reagent preparation approaches reported to date.


 Please wait while we load your content...
Please wait while we load your content...