In situ generation of radical initiators using amine-borane complexes for carbohalogenation of alkenes†
Abstract
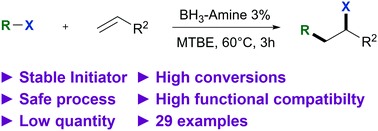
Atom transfer radical addition of alkyl halides to alkenes was developed using a low amount of a stable initiator, amine borane complexes. Thanks to a slow hydroboration step, the overall carbohalogenation process leads to good isolated yields.
- This article is part of the themed collection: Boron Chemistry in the 21st Century: From Synthetic Curiosities to Functional Molecules


 Please wait while we load your content...
Please wait while we load your content...