Abstract
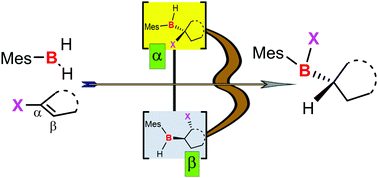
The direct access to (mesityl)(alkyl)haloboranes (Mes(Alk)BX) (X = Br, Cl) from mesitylborane dimer and vinyl halides is presented. The involved hydroboration reaction results in the transfer of the halogen atom from the carbon of the starting material to the boron in the final product. The reactivity of the obtained Mes(Alk)BX has been evaluated for the synthesis of the bipyridyl boronium cations and 2-arylpyridine derived boron N^C-chelates. The formation mechanism of Mes(Alk)BX is apprended by DFT-calculations which shows that their formation involves two concomitant pathways derived from the regioslectivity of the hydroboration reaction.
- This article is part of the themed collection: Boron Chemistry in the 21st Century: From Synthetic Curiosities to Functional Molecules


 Please wait while we load your content...
Please wait while we load your content...