Development of chiral potassium strong Brønsted base catalysts for enantioselective carbon–carbon bond-forming reactions
Abstract
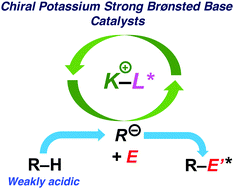
Chiral alkaline metal Brønsted bases are traditional and reliable promoters in enantioselective catalysis. Here, new chiral potassium strong base catalysts were developed for enantioselective carbon–carbon bond-forming reactions of weakly acidic carbon pronucleophiles. Chiral potassium amide or alkyl potassium catalyzed enantioselective addition reactions to imines or α,β-unsaturated amides with good to high enantioselectivities. The good potential of chiral potassium Brønsted bases to act as proton transfer catalysts has been shown.


 Please wait while we load your content...
Please wait while we load your content...