Iodine-mediated electrochemical C(sp3)–H cyclization: the synthesis of quinazolinone-fused N-heterocycles†
Abstract
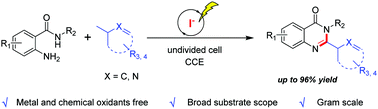
An efficient iodine-mediated electrochemical C(sp3)–H cyclization was developed under mild conditions. A variety of functionalized quinazolinone-fused N-heterocycles can be obtained with good to excellent yields by virtue of this method. The reaction features a broad substrate scope and scalability, and is metal-free and chemical oxidant-free.


 Please wait while we load your content...
Please wait while we load your content...