Abstract
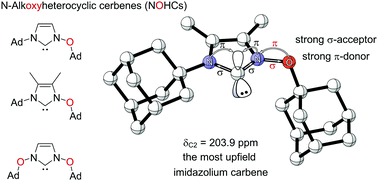
We report the first preparation of N-alkoxyimidazolylidene (NOHC), a nucleophilic carbene based on an oxidized imidazolium core. The Arduengo-type analogous carbene center shows the most upfield 13C NMR shift compared to common NHCs. The obtained gold(I) complex of the carbene follows the 13C NMR upfield trend and shows the marked influence the alkoxy substituents. Similarly, the 77Se and 15N NMR shifts of a range of NOHC-selenium adducts show increased σ-donation and decreased π-back donation in the bonding with the nucleophile. This extension of the NHC family provides altered electronic properties for the use of such carbenes as ligands or catalysts.


 Please wait while we load your content...
Please wait while we load your content...