Rubidium and caesium aluminyls: synthesis, structures and reactivity in C–H bond activation of benzene†
Abstract
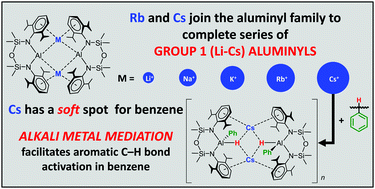
Expanding knowledge of low valent aluminium chemistry, rubidium and caesium aluminyls are reported to complete the group 1 (Li–Cs) set of metal aluminyls. Both compounds crystallize as a contacted dimeric pair supported by M⋯π(arene) interactions with a pronounced twist between aluminyl units. Density functional theory calculations show symmetrical bonding between the M and Al atoms, with an Al centred lone-pair donating into vacant Rb and Cs orbitals. Interestingly, despite their structural similarity the Cs aluminyl enables C–H bond activation of benzene, but not the Rb aluminyl reflecting the importance of the alkali metal in these heterobimetallic systems.


 Please wait while we load your content...
Please wait while we load your content...