The improved killing of both androgen-dependent and independent prostate cancer cells by etoposide loaded SPIONs coupled with NIR irradiation†
Abstract
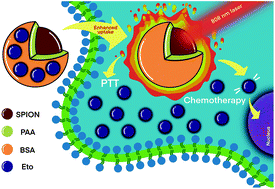
Etoposide (Eto) is a toxic drug that shows promise in treating prostate cancer (PCa) but confers significant side effects, and has poor solubility and bioavailability. Nanoparticles are quite successful in overcoming such problems. Multifunctional nanoparticles that provide an opportunity to perform combination therapy have attracted great interest in recent years. Superparamagnetic iron oxide nanoparticles (SPIONs) are popular in various biomedical applications, including magnetic resonance imaging, drug delivery, magnetic hyperthermia and recently in photothermal therapy, combining imaging with therapy. Here, for the enhanced killing of PCa cells that are either androgen-dependent or not, the combination of SPION based Eto delivery and mild hyperthermia triggered by laser irradiation is proposed for the first time in the literature. For the encapsulation of Eto, highly stable, small, polyacrylic acid coated SPIONs were conjugated with bovine serum albumin (BSA) (Eto-BSA@PAA@SPION). Eto-BSA@PAA@SPION with 9% drug content produced better chemotherapeutic outcomes than free Eto on both androgen-dependent/castration sensitive LNCaP and androgen-independent/castration-resistant PC3 and DU145 PCa cells by enhancing drug internalization. Single and short irradiation of Eto-BSA@PAA@SPION treated cells at 808 nm improved the drug release and sensitized cells for Eto, hence, increasing the toxicity dramatically in all studied PCa cell lines. Caspase-mediated apoptosis, DNA damage, and ROS generation were detected in the treated cells, increasing with the Eto dose and laser treatment. The IC50 for Eto is reduced to 0.08 μg mL−1, 0.13 μg mL−1 and 2.8 μg mL−1 with laser/Eto-BSA@PAA@SPION for LNCaP, DU145 and PC3 cells, respectively. These are the lowest IC50 values seen in the literature for Eto on these cell lines so far, suggesting that the demonstrated nanoparticles and treatment approaches have great potential to treat various PCa cells at low doses of the drug under mild laser treatment conditions.


 Please wait while we load your content...
Please wait while we load your content...