Magnetic nanocluster-mediated photothermal effect and macrophage modulation for synergistic photothermal immunotherapy of cancer†
Abstract
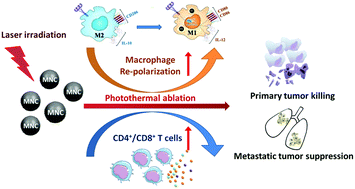
In the tumor microenvironment, macrophages predominately exhibit M2-type functionalities which promote malignant progression and cancer metastasis, thus posing a big hurdle for current anticancer strategies. Different approaches have been exploited to reverse the macrophages towards the M1 pro-inflammatory phenotype; however, it is hard to achieve tumor regression with this macrophage modulation alone. Herein we synthesized photothermal magnetic nanoclusters (MNCs) to test their capability for reprograming M2 macrophages towards the M1 phenotype. We demonstrated that these photothermal MNCs themselves can effectively trigger this desired macrophage repolarization, increase the phagocytosis of macrophages at the tumor site, and cause subsequent T cell activation with enhanced systemic cytokine release, leading to cancer cell killing both in vitro and in vivo. More interestingly, it was found that the photothermal effect can further facilitate this immune activation to a greater extent, inducing much higher level of macrophage repolarization and T cell infiltration into the tumor site as well as eliciting a much more efficient antitumor effect compared with MNCs alone. Our findings demonstrate that MNCs combined with their photothermal effect can reverse the local suppressive environment of the tumor by macrophage and T cell modulation, providing an effective approach to trigger systemic immune responses that contribute to an enhanced overall anticancer outcome and the suppression of cancer metastasis.


 Please wait while we load your content...
Please wait while we load your content...