Naphthalene-functionalized resorcinarene as selective, fluorescent self-quenching sensor for kynurenic acid†
Abstract
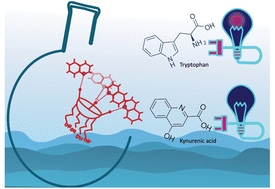
Kynurenic acid is a by-product of tryptophan metabolism in humans, with abnormal levels indicative of disease. There is a need for water-soluble receptors that selectively bind kynurenic acid, allowing for detection and quantification. We report here the high-affinity binding of kynurenic acid in aqueous media to a resorcinarene salt receptor decorated with four flexible naphthalene groups at the upper rim. Experimental results from 1H NMR, isothermal titration calorimetry, and electronic absorption and fluorescence spectroscopies all support high-affinity binding and selectivity for kynurenic acid over tryptophan. The measured binding constant (K = 1.46 ± 0.21 × 105 M−1) is one order of magnitude larger than that observed with other resorcinarene receptors. The present host–guest system can be employed for sensory recognition of kynurenic acid. Computational studies reveal the key role of a series of cooperative attractive intra- and inter-molecular interactions contributing to an optimal binding process in this system.


 Please wait while we load your content...
Please wait while we load your content...