Shielding effect and compensation defect study on Na3Sc2(PO4)y:Eu2+,3+ (y = 2.6–3.0) phosphor by anion-group-induced phase transition†
Abstract
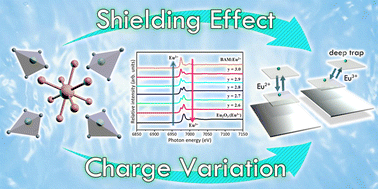
Due to the excellent thermal property of Na superionic conductors (NASICON), research has focused on studying crystal structure parameters under high temperature conditions. In this study, different ratios of anion groups were regulated to induce vacancy defects on the Eu-doped phosphate phosphor Na2.87Sc2(PO4)y:0.13Eu2+, y = 2.6–3.0. When the amount of the anion group increased, a structural transformation was induced from γ to β-phase. Using the X-ray absorption near edge structure (XANES) technique, this phenomenon was found to be caused by Eu3+ and Eu2+ coexisting due to the amount of anion group. It is rare to observe this situation with 2D X-ray fluorescence (XRF) technology, with direct evidence of the shielding effect provided by XRF mapping. We speculate that the oxidation reaction creates trivalent europium due to the lack of the –PO4 anion group to protect the divalent europium (Eu2+) and we explain the luminescence mechanism by a charge compensation model. Thermal stability was maintained at 90% from room temperature to 150 °C. The pathway of the reduction mechanism and the location of the ground level from Eu2+ (β-phase) to Eu3+ (γ-phase) are evidenced by high-pressure spectroscopy. Finally, this study proposes a generic path for the anion group phosphor research.


 Please wait while we load your content...
Please wait while we load your content...