Cross-conjugated isothianaphthene quinoids: a versatile strategy for controlling electronic structures†
Abstract
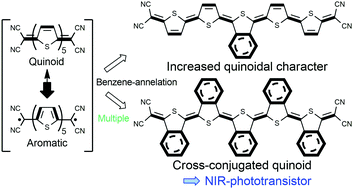
The elucidation of new structure–property relationships in π-conjugated molecules bearing quinoidal moieties is of relevance because of their use in organic electronics applications and their traditional assimilation as models of doped conducting polymers. Quinoidal oligothiophenes are ground state electronic hybrids between closed-shell Kekulé quinoidal and open-shell aromatic diradicaloid forms. The prominent contribution of the diradical character in longer oligomers beyond thiophene 4-mers results in a low stability, thereby limiting the ability to tune their properties. Thus, the control of these quinoidal/aromatic contributions is an important prerequisite to develop long quinoidal oligothiophenes. To address this problem, a series of quinoidal pentathiophenes with benzene-annelated isothianaphthene units were designed and successfully synthesized as stable structures. Combined molecular spectroscopies and theoretical modelling indicated that cross-conjugation appears upon the introduction of multiple benzene-annelated units, and that the number and position of the benzene-annelated units have a significant influence on the quinoidal/aromatic/cross-conjugated electronic structures. The newly developed quinoidal pentathiophenes functioned as organic semiconducting materials in transistor and near infrared phototransistor devices. This study demonstrates that modification of the cross-conjugated quinoidal structure is a promising strategy for fine-tuning electronic structures in π-extended quinoidal systems, which could help us to understand unique π-electronic features and to develop novel organic electronic materials.


 Please wait while we load your content...
Please wait while we load your content...