Revisiting syntheses of Fe3O4 nanoparticles in water and lower alcohols and their resistive switching properties†
Abstract
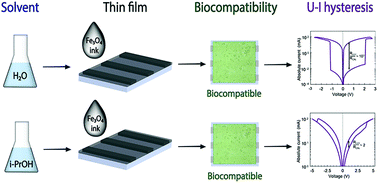
Magnetite (Fe3O4) nanoparticles have found numerous applications due to the ease of fabrication, favourable combination of physical and chemical properties, as well as environmental and biological safety. At the same time, their functional applications in memristive devices remain underexplored, especially with regard to nanocrystalline phases that can be obtained by various means of solution chemistry. In this study, we examine the physical properties, morphology, and biocompatibility of magnetite nanoparticles obtained by hydrolytic and non-hydrolytic synthesis routes. For this purpose, we have revisited two solution chemistry methods for obtaining magnetite nanoparticles in water and lower alcohols. Notably, magnetite nanoparticles obtained via the hydrolytic route have demonstrated an appreciably high value of the resistive switching ratio ROFF/RON ∼ 103 which is comparable to the highest values ever reported for iron oxide memristors. The advantageous suitability of the hydrolytically synthesized magnetite nanoparticles for resistive switching applications is rationalized by their higher purity and crystallinity, as well as by the plausible activity of residual water molecules and hydroxy groups on facilitating the topotactic redox reaction associated with the resistive switching phenomenon.


 Please wait while we load your content...
Please wait while we load your content...