Thiolated cationic poly(aspartamides) with side group dependent gelation properties for the delivery of anionic polyelectrolytes†
Abstract
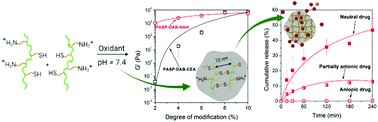
In situ gellable polymers have potential applications as injectable formulations in drug delivery and regenerative medicine. Herein, thiolated cationic polyaspartamides were synthesised via two different approaches to correlate the side group structure with gelation properties, gel strength and drug release kinetics. Cysteamine (CEA) was used as a thiolating agent to prepare thiolated cationic polyaspartamide groups with short thiolated side groups. As a new pathway, thiolactone chemistry was integrated with cationic modification of polyaspartamides to prepare thiolated derivatives with longer, flexible side groups using N-acetyl-DL-homocysteine (NAH) thiolactone. Both types of thiolated polyaspartamides could be converted into stiff hydrogels under mild reaction conditions through oxidation-induced intermolecular disulfide formation. We confirmed that the longer side groups largely accelerated gelation and the stiffness of the resultant hydrogels was higher than that of the CEA-modified counterparts. Both the gelation time and stiffness could be adjusted by the degree of thiolation. Poly(aspartic acid) (PASP) derivatives with a controlled concentration of anionic groups were entrapped in the hydrogels during the in situ gelation. Based on the possible electrostatic interaction between the linear anionic polyelectrolytes and the cationic polymer network, we hypothesized that the release of the encapsulated material is controlled by the charge density. In accordance, fully anionic PASP was entrapped completely in the hydrogels, whereas a reduction in the number of anionic groups caused the partial release of PASP derivatives. NAH- and CEA- modified cationic polyaspartamide hydrogels showed distinct release rates, indicating the interplay between cationic and thiol functionalities in release kinetics.

 Please wait while we load your content...
Please wait while we load your content...