Cross-linked proton-exchange membranes with strongly reduced fuel crossover and increased chemical stability for direct-isopropanol fuel cells
Abstract
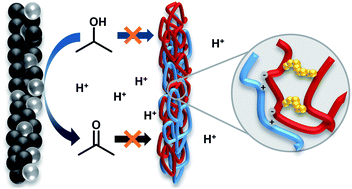
Isopropanol fuel cells offer an attractive way to provide electric energy from a liquid, easily transportable fuel without producing significant amounts of CO2. The oxidation product acetone can be easily hydrogenated back to isopropanol to close the storage cycle, thereby avoiding the sophisticated handling of fugitive molecular hydrogen. Until now, direct-isopropanol fuel cells (DIFC) usually rely on various perfluorosulfonic acid ionomers, like Nafion, which are costly and have an unfavorable high fluorine content. Additionally, the dissolution of Nafion in isopropanol/acetone/water solutions within respective applications has prevented the long time operation of DIFCs so far. The swelling of those ionomers during operation promotes fuel crossover and reduces the system's overall energy efficiency. This study uses ionic cross-linking of polymer blends to manufacture chemically stable membranes and introduces a new click-like covalent cross-linking strategy for ion exchange polymers. Compared to Nafion XL, the manufactured membranes increase the maximum power density by up to 10%, resist a dissolution stress test up to 84 w% and reduce the detected isopropanol/acetone crossover up to 75/100% during fuel cell operation. Consequently, the material can be considered a major step toward the technical implementation of isopropanol fuel cell technologies.


 Please wait while we load your content...
Please wait while we load your content...