Selecting the best dopant sites in proton-conducting pyrochlore oxides (La2(Nb1−xYx)2O7−δ) by probing hydration-induced local distortion†
Abstract
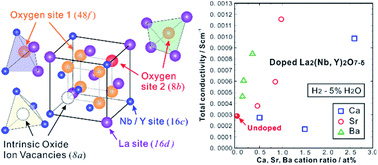
Electrolytes with excellent chemical stability and high proton conductivity are key materials for protonic ceramic fuel cells and electrolyzers. Doping aliovalent cations to increase the number of oxygen vacancies is a common method to increase conductivity. However, determining proper sites for efficient doping is a matter of general concern because in many cases, there are several cation sites in complex oxides. In this work, we used EXAFS analysis to probe the changes in the local structure of proton-conducting pyrochlore oxides, La2(Nb0.4Y0.6)2O7−δ (LNY60) and La2(Nb0.35Y0.65)2O7−δ (LNY65), upon hydration and found that the coordination number of the oxygen sites in the vicinity of La and Y increased after hydration, indicating that these oxygen sites were active for the hydration reaction to introduce protons. Thereafter, the trivalent La sites were doped with divalent Ca, Sr and Ba cations to introduce more oxygen vacancies into the adjacent oxygen sites to facilitate the hydration reaction. As expected, the proton conductivity increased with increasing content of Ca, Sr and Ba. The conductivities of 2.54 at% Ca-doped LNY60 and 0.98 at% Sr-doped LNY65 were both 1.16 × 10−3 S cm−1 at 700 °C, nearly four times higher than those of the pristine oxides. These results indicate that assisted by EXAFS analysis, proper dopant sites can be determined precisely to further elevate proton conductivity in oxides.


 Please wait while we load your content...
Please wait while we load your content...