High-corrosion-resistance mechanism of graphitized platelet-type carbon nanofibers in the OER in a concentrated alkaline electrolyte†
Abstract
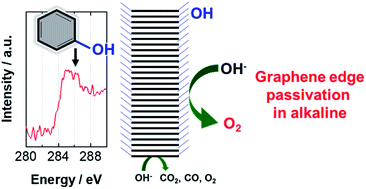
Carbon materials are used as electrocatalyst supports and conductive additives in various electrodes for electrochemical energy conversion and storage because of their high electrical conductivity and chemical stability. However, they suffer from electrochemical corrosion at high anodic potentials in aqueous electrolytes. This study demonstrates that highly graphitized platelet-type carbon nanofibers (pCNFs) are tolerant to electrochemical corrosion under oxygen evolution reaction (OER) conditions in a concentrated KOH electrolyte. An identical-location scanning electron microscopy study showed that the corrosion rate of the carbon edge plane of the pCNFs is very low compared with that of the carbon basal plane. Further scanning transmission electron microscopy/electron-energy loss spectroscopy studies indicate that the carbon edge plane of the pCNFs is covered with hydroxyl groups, whereas the oxygen-containing species are limited to the carbon basal plane. Thus, most surfaces of highly graphitized pCNFs, consisting of the carbon edge plane, are passivated with hydroxyl groups, resulting in the high resistance of pCNFs to electrochemical corrosion. These findings provide a novel material design for carbon with a high corrosion resistance in the OER.


 Please wait while we load your content...
Please wait while we load your content...