Instability of Ga-substituted Li7La3Zr2O12 toward metallic Li†
Abstract
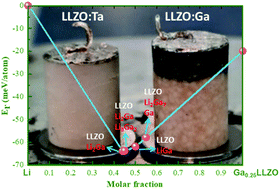
Ga-substituted Li7La3Zr2O12 (LLZO) garnet is among the most promising solid electrolytes for next-generation all-solid-state Li battery (SSLB) applications due to its very high Li-ion conductivity. However, the attempts to use Ga-substituted LLZO as a solid electrolyte for SSLBs are not yet successful. Here, the research results show that Li6.4Ga0.2La3Zr2O12 can be reduced by Li at 25 °C when the surface of the material is properly cleaned. The experimental results suggest that Ga leached out of the garnet structure to form the Li–Ga alloy, which apparently would short-circuit the battery if Ga-substituted LLZO is used as a solid electrolyte. When low concentration Ga-substitution is applied, e.g. Li6.45Ga0.05La3Zr1.6Ta0.4O12, the material seems stable against Li at ambient temperature but not at high temperatures, where heat treatment is usually used to reduce the interfacial resistance between Li and LLZO. The experimental results are also supported by density functional theory calculations to show that the Ga-substituted LLZO/Li interface tends to transform into LLZO and the Li2Ga intermetallic compound. The results highlight the importance of substitution selection for LLZO, for which the Ga-substituted LLZO solid electrolyte may not be suitable for direct contact with metallic Li.


 Please wait while we load your content...
Please wait while we load your content...