Fluoride-ion conversion alloy for fluoride-ion batteries†
Abstract
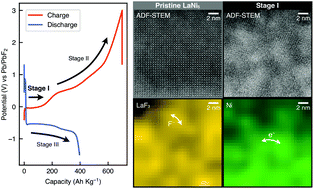
To realize the full potential of fluoride-ion batteries with a significantly high energy density (larger than 1000 W h kg−1), it is a prerequisite to develop another conceptual cathode material beyond the current pure metals. This is because pure metals can only be fluorinated on the surface with typically less than 10 nm depth. To overcome this issue, we show here that well-established hydrogen storage alloys such as LaNi5 could be promising candidates for cathode materials in fluoride-ion batteries, where the reversible capacity of LaNi5 is 396 A h kg−1. By using atomic-resolution scanning transmission electron microscopy, we elucidate that LaNi5 particles are first decomposed into LaF3 and Ni nanocrystals in the charge process. A nano-scale network is formed by LaF3 and Ni nanocrystals during the charge process, which maintains F−-ion and electric conductivities in the particles. Furthermore, Ni – one of the most difficult elements to fluorinate – can be fluorinated and defluorinated in the subsequent charge and discharge processes, because of the spontaneous formation of Ni nanocrystals which are smaller than the passivation layer thickness and LaF3 nano-scale network. Our results suggest that an intermetallic alloy containing two function-sharing elements could be useful to explore higher capacity cathodes in fluoride-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...