Revealing the activity of Co3Mo3N and Co3Mo3N0.5 as electrocatalysts for the hydrogen evolution reaction†
Abstract
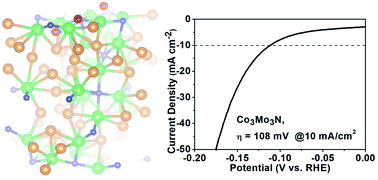
The hydrogen evolution reaction (HER) from water is governed by the electrocatalysts used. Multiple factors such as crystal structure, composition and morphology dictate the final catalytic performance. However, as multicomponent materials are developed to replace noble metals in the HER, it has become increasingly difficult to identify intrinsically active materials. Hence, there is an imperative for phase-pure catalysts to be synthesized and tested without obscuring contributions from impurities or substrates. Herein, we demonstrate that phase-pure, unsupported Co3Mo3N achieves a competitively low overpotential (OVP) of 108 ± 8 mV at 10 mA cm−2 in 0.5 M H2SO4. Density functional theory (DFT) reveals weakly binding metal sites as the catalytic centres for the HER in the nitride. Remarkably, the N-deficient Co3Mo3N0.5 shows similar electrochemical properties but has limited chemical stability under cathodic bias. Thus, even though nitrogen sites play only a minor role in catalytic performance, their occupancy is crucial for the stability of nitride catalysts in the corrosive electrolyte. The composite of Co3Mo3N on Ni-foam sustains 10 ± 1 mA cm−2 at an applied potential of just 20 mV over extended time, highlighting the utility of nitrides for the future design of stable and active HER catalytic systems.


 Please wait while we load your content...
Please wait while we load your content...