Nitrogen-induced interfacial electronic structure of NiS2/CoS2 with optimized water and hydrogen binding abilities for efficient alkaline hydrogen evolution electrocatalysis†
Abstract
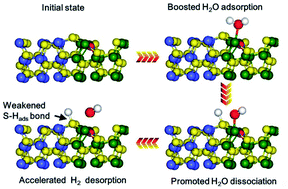
Fabricating heterostructures with dense interfacial catalytic sites is essential for implementation of high-performance hydrogen evolution reaction (HER). However, the strong correlation between adsorbed hydrogen atoms and electronegative nonmetal atoms gives rise to the unfavorable hydrogen desorption behavior on the heterointerface, especially on the metal sulfide heterointerface. Herein, we present a simple way to incorporate nitrogen into the NiS2/CoS2 heterostructure (N–NiS2/CoS2) to form an electron-reconfiguration heterointerface. The electronic structure of active sites on the N–NiS2/CoS2 interface can be effectively tuned by N doping, thus realizing a promoted water adsorption and dissociation capability, and near-optimal hydrogen desorption behavior on the N–NiS2/CoS2 interface. Consequently, the as-prepared N–NiS2/CoS2 heterointerface demonstrates an impressive HER performance with a low overpotential of 73 mV at a current density of 10 mA cm−2 and a small Tafel slope of 65 mV dec−1. This work sheds light on the positive effects of interfacial element dopants on the HER performance, and also provides valuable insights for rational design of a high-performance heterointerface electrocatalyst.


 Please wait while we load your content...
Please wait while we load your content...