CO reductive oligomerization by a divalent thulium complex and CO2-induced functionalization†
Abstract
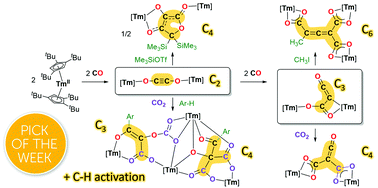
The divalent thulium complex [Tm(Cpttt)2] (Cpttt = 1,2,4-tris(tert-butyl)cyclopentadienyl) reacts with CO to afford selective CO reductive dimerization and trimerization into ethynediolate (C2) and ketenecarboxylate (C3) complexes, respectively. DFT calculations were performed to shed light on the elementary steps of CO homologation and support a stepwise chain growth. The attempted decoordination of the ethynediolate fragment by treatment with Me3SiI led to dimerization and rearrangement into a 3,4-dihydroxyfuran-2-one complex. Investigation of the reactivity of the C2 and C3 complexes towards other electrophiles led to unusual functionalization reactions: while the reaction of the ketenecarboxylate C3 complex with electrophiles yielded new multicarbon oxygenated complexes, the addition of CO2 to the ethynediolate C2 complex resulted in the formation of a very reactive intermediate, allowing C–H activation of aromatic solvents. This original intermolecular reactivity corresponds to an unprecedented functionalization of CO-derived ligands, which is induced by CO2.
- This article is part of the themed collections: 2022 ChemSci Pick of the Week Collection and 2022 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...