Benzoates as photosensitization catalysts and auxiliaries in efficient, practical, light-powered direct C(sp3)–H fluorinations†
Abstract
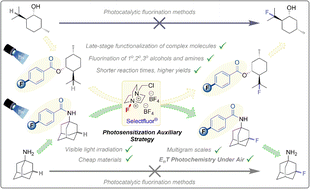
Of the methods for direct fluorination of unactivated C(sp3)–H bonds, photosensitization of SelectFluor is a promising approach. Although many substrates can be activated with photosensitizing catalysts, issues remain that hamper fluorination of complex molecules. Alcohol- or amine-containing functional groups are not tolerated, fluorination regioselectivity follows factors endogenous to the substrate and cannot be influenced by the catalyst, and reactions are highly air-sensitive. We report that benzoyl groups serve as highly efficient photosensitizers which, in combination with SelectFluor, enable visible light-powered direct fluorination of unactivated C(sp3)–H bonds. Compared to previous photosensitizer architectures, the benzoyls have versatility to function both (i) as a photosensitizing catalyst for simple substrate fluorinations and (ii) as photosensitizing auxiliaries for complex molecule fluorinations that are easily installed and removed without compromising yield. Our auxiliary approach (i) substantially decreases the reaction's induction period, (ii) enables C(sp3)–H fluorination of many substrates that fail under catalytic conditions, (iii) increases kinetic reproducibility, and (iv) promotes reactions to higher yields, in shorter times, on multigram scales, and even under air. Observations and mechanistic studies suggest an intimate ‘assembly’ of auxiliary and SelectFluor prior/after photoexcitation. The auxiliary allows other EnT photochemistry under air. Examples show how auxiliary placement proximally directs regioselectivity, where previous methods are substrate-directed.


 Please wait while we load your content...
Please wait while we load your content...