Heteroarylation of unactivated C–H bonds suitable for late-stage functionalization†
Abstract
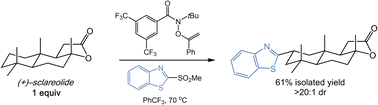
The late-stage introduction of diverse heterocycles onto complex small molecules enables efficient access to new medicinally relevant compounds. An attractive approach to such a transformation would utilize the ubiquitous aliphatic C–H bonds of a complex substrate. Herein, we report a system that enables direct C–H heteroarylation using a stable, commercially available O-alkenylhydroxamate with heterocyclic sulfone partners. The C–H heteroarylation proceeds efficiently with a range of aliphatic substrates and common heterocycles, and is a rare example of heteroarylation of strong C–H bonds. Importantly, the present approach is amenable to late-stage functionalization as the substrate is the limiting reagent in all cases.


 Please wait while we load your content...
Please wait while we load your content...