Critical stresses in mechanochemical reactions†
Abstract
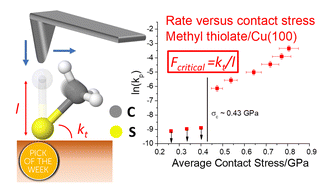
The rates of mechanochemical reactions are generally found to increase exponentially with applied stress. However, a buckling theory analysis of the effect of a normal stress on an adsorbate that is oriented perpendicularly to the surface that reacts by tilting suggests that a critical value of the stress should be required to initiate a mechanochemical reaction. This concept is verified by using density functional theory calculations to simulate the effect of compressing a homologous series of alkyl thiolate species on copper by a hydrogen-terminated copper counter-face. This predicts that a critical stress is indeed needed to initiate methyl thiolate decomposition, which has a perpendicular C–CH3 bond. In contrast, no critical stress is found for ethyl thiolate with an almost horizontal C–CH3 bond, while a critical stress is required to isomerize propyl thiolate from a trans to a cis configuration. These predictions are tested by measuring the mechanochemical reaction rates of these alkyl thiolates on a Cu(100) substrate by sliding an atomic force microscope tip over the surface and finding a critical stress of ∼0.43 GPa for methyl thiolate, ∼0.33 GPa for propyl thiolate, but no evidence of a critical stress for ethyl thiolate, in accord with the predictions. These results provide insights not only into mechanochemical reaction mechanisms on surfaces, but also on the origin of critical phenomena in stress-induced processes in general. It also suggests novel approaches to designing robust surface films that can resist wear and damage.
- This article is part of the themed collections: 2022 ChemSci Pick of the Week Collection and 2022 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...