N-Cyanorhodamines: cell-permeant, photostable and bathochromically shifted analogues of fluoresceins†
Abstract
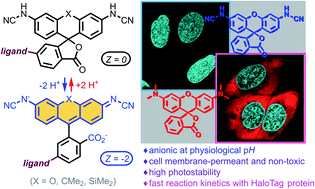
Fluorescein and its analogues have found only limited use in biological imaging because of the poor photostability and cell membrane impermeability of their O-unprotected forms. Herein, we report rationally designed N-cyanorhodamines as orange- to red-emitting, photostable and cell-permeant fluorescent labels negatively charged at physiological pH values and thus devoid of off-targeting artifacts often observed for cationic fluorophores. In combination with well-established fluorescent labels, self-labelling protein (HaloTag, SNAP-tag) ligands derived from N-cyanorhodamines permit up to four-colour confocal and super-resolution STED imaging in living cells.
- This article is part of the themed collection: 2022 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...