Sustainable and highly controlled aryl couplings revealed by systematic assessment of photoactivatable linkers†
Abstract
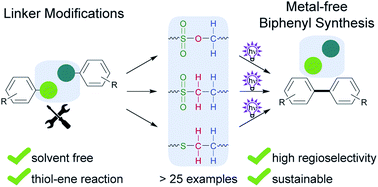
The controlled synthesis of biphenyls, which play a prominent role in pharmaceuticals, agrochemicals, and liquid crystals, typically requires hazardous organometallic reagents, aryl halides, and heavy metal catalysts. We recently reported a metal-free, photochemical alternative (“photosplicing”) for the selective preparation of a wide range of pharmaceutically important biphenyls. Whereas the traceless sulfonamide linker enables and controls the aryl coupling, unwanted toxic byproducts are released. Therefore, we designed over 25 different temporary linkers and tested them for their suitability for the photosplicing reaction in a flow reactor. We found that a surprisingly high number of functional groups enable light-induced aryl fusion and identified a number of linkers for environmentally friendly procedures. We also report that a thiol-ene (click) – photosplicing sequence enables a convenient route to biaryls such as liquid crystals. This work sheds light on thus far neglected photochemistry of temporary linkers, reduces toxic byproducts, and expands the available starting materials for metal-free biphenyl synthesis.


 Please wait while we load your content...
Please wait while we load your content...