An expeditious route to sterically encumbered nonproteinogenic α-amino acid precursors using allylboronic acids†
Abstract
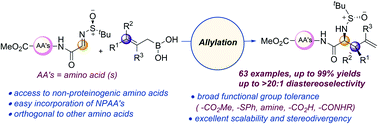
A diastereoselective allylation of N-tert-butane sulfinyl α-iminoesters using allylboronic acids is developed to obtain optically active non-proteinogenic α-amino acid precursors in good yields and diastereoselectivities. Gram-scale synthesis, broad tolerance of functional groups, excellent stereodivergence, post-synthetic modifications, and easy removal of the chiral auxiliary are some of the key highlights. The protocol is applicable to various amino acids and short peptides, resulting in the incorporation of these precursors at the N-terminal position.


 Please wait while we load your content...
Please wait while we load your content...