Growth kinetics determine the polydispersity and size of PbS and PbSe nanocrystals†
Abstract
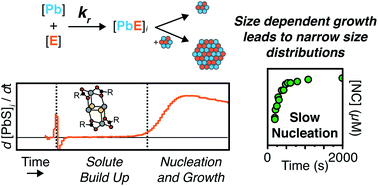
A library of thio- and selenourea derivatives is used to adjust the kinetics of PbE (E = S, Se) nanocrystal formation across a 1000-fold range (kr = 10−1 to 10−4 s−1), at several temperatures (80–120 °C), under a standard set of conditions (Pb : E = 1.2 : 1, [Pb(oleate)2] = 10.8 mM, [chalcogenourea] = 9.0 mM). An induction delay (tind) is observed prior to the onset of nanocrystal absorption during which PbE solute is observed using in situ X-ray total scattering. Density functional theory models fit to the X-ray pair distribution function (PDF) support a Pb2(μ2-S)2(Pb(O2CR)2)2 structure. Absorption spectra of aliquots reveal a continuous increase in the number of nanocrystals over more than half of the total reaction time at low temperatures. A strong correlation between the width of the nucleation phase and reaction temperature is observed that does not correlate with the polydispersity. These findings are antithetical to the critical concentration dependence of nucleation that underpins the La Mer hypothesis and demonstrates that the duration of the nucleation period has a minor influence on the size distribution. The results can be explained by growth kinetics that are size dependent, more rapid at high temperature, and self limiting at low temperatures.


 Please wait while we load your content...
Please wait while we load your content...